 Two
physicists have experimentally determined the melting point of iron in Earth’s
core. New data from the highest pressure experiments to date show that iron
begins to melt at a lower pressure than previously thought and that a second
crystalline form of solid iron — theorized for the past 20 years to occur
in the core — does not exist.
Two
physicists have experimentally determined the melting point of iron in Earth’s
core. New data from the highest pressure experiments to date show that iron
begins to melt at a lower pressure than previously thought and that a second
crystalline form of solid iron — theorized for the past 20 years to occur
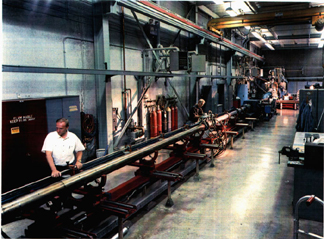
in the core — does not exist.Physicists are using a giant gas gun at Lawrence Livermore National Laboratory in Livermore, Calif., to smash an iron bullet into an iron target at high velocity — creating a shockwave and producing extreme pressures for studying conditions at Earth’s core. Photo courtesy of Lawrence Livermore National Laboratory.
“What we have determined is that the solid-solid phase transition doesn’t exist,” says Jeffrey Nguyen, a physicist from Lawrence Livermore National Laboratory (LLNL) in Livermore, Calif., “and that the pressure that iron melts at is lower than previously reported.”
The findings are a step forward in the ongoing effort to accurately describe how iron behaves at high pressures and temperatures. Working out the extremes of iron’s melting curve is key to understanding the thermodynamics of the planet, especially in the planet’s core, where liquid iron swirling in the outer core generates Earth’s geomagnetic field.
As reported in the Jan. 22 Nature, Nguyen and LLNL colleague Neil Holmes found that iron under core conditions starts to melt at 225 Gigapascals (32 million pounds per square inch) and a temperature of 5,100 degrees Kelvin (about 4,800 degrees Celsius), and finishes melting by 260 Gigapascals at a temperature of 6,100 degrees Kelvin. Those values fall between the pressures that are known from seismological surveys to exist at the liquid outer core’s boundary.
The experimental values were also very close to theoretical values calculated by Dario Alfè and colleagues at University College London. “We believe that this is good news,” Alfè says.
For years, scientists have been attempting, both theoretically and experimentally, to plot and connect new points on the high-pressure, high-temperature end of iron’s phase diagram in order to reveal iron’s melt line. They have not been able to reach a consensus, however, and estimates of iron’s melting point at pressures found in the core have differed by 2,000 degrees Celsius or more.
“Trying to work out what happens at pressures of millions of atmospheres — the pressures inside Earth’s core — is by no means an easy task, and experiments are extremely challenging,” Alfè says.
The only way to create and study high-pressure core conditions, Alfè says, is through experiments, in which an iron bullet is smashed into an iron target at high velocity to create a shockwave. As with the core, the temperature of iron in the shockwave experiments cannot be measured directly. Instead, researchers record the changes in pressure, volume and sound velocity that occur on impact. “What we do is look at how sound velocity changes with pressure,” Nguyen says.
To create a shockwave in the target, the researchers used a 20-meter-long two-stage gas gun that can accelerate a bullet up to 8 kilometers per second and create impact pressures exceeding 400 Gigapascals (58 million pounds per square inch). “This is the highest pressure on the iron melt curve anyone has ever gotten to,” Nguyen says. The pressure at the center of Earth is 360 Gigapascals.
Although the conditions exist for only a millionth of a second, it is long enough for researchers to measure the shockwaves traveling through the target. The experiment is repeated at different pressures until a drop in sound velocity indicates a phase change.
Researchers then face the additional challenge of calculating the melting temperature using a thermodynamic equation that involves two variables whose values are not known exactly. “If you get these two parameters wrong, you also get the melting temperature wrong,” Alfè says. “For this reason experiments based on this technique have been criticized for a long time.”
The Livermore researchers, however, used the same values for these parameters as earlier experiments, including the study that found two solid phases of iron 20 years ago. Alfè notes the variables also are close to theoretical values, as are the resulting pressures and calculated melting temperature.
“Our calculated values are very close to those used by Nguyen and Holmes, therefore supporting their results,” Alfè says. “But still more work is needed to confirm and extend these recent findings.”

