geotimesheader
News Notes
Planetary
geology
More
like Earth than ever before
Scientists have known for decades that Earth’s interior is differentiated
and that its core is primarily iron. But only recently have they been able
to determine the interior compositions of other planetary bodies.
Remote sensing data from the Hubble Space Telescope and the Galileo
spacecraft offer valuable information about Io's gravitational field and
the chemical composition of its volcanic plumes. Recent research shows
that this jovian moon, like Earth, has an iron core surrounded by concentric
zones of iron-depleted, heterogeneous material. Io is a present day analogue
for ancient Earth. |

A color-enhanced Galileo image
of Jupiter's
moon Io. NASA |
The Galileo mission gave Gerald Schubert of the University of California
at Los Angeles the gravitational data he needed to determine that Io has
an iron core. As Galileo flew by Io for the first time in December 1995,
Io's gravitational pull caused Galileo to change velocity as it
moved toward and away from the moon. The signals received on Earth reflected
Galileo’s velocity change and allowed Schubert to determine Io’s
gravitational field. With that, Schubert could determine Io’s mass. Knowing
the size of Io’s equatorial bulge, he calculated the moon’s moment of inertia.
The ratio of the moment of inertia to the mass, multiplied by the square
of the radius, is constant for a homogenous body. Schubert calculated that
the ratio for Io is less than the constant value of a homogenous body,
revealing that Io must have a concentration of mass at its center. “Iron
is the only element sufficiently heavy and abundant in the solar system
to account for a large density of mass at the center of Io,” Schubert says.
Using data collected by the Hubble Space Telescope over a span of less
than an hour, scientists Mikhail Zolotov and Bruce Fegley of Washington
University in St. Louis were able to model the eruptive conditions of Pele
— Io’s most active volcano — based on the chemical composition of Pele’s
volcanic plume. Zolotov and Fegley published their work in the September
Geophysical Research Letters.
Where gravitational analyses cannot determine the extent to which the
mantle is iron-depleted, Zolotov and Fegley’s chemical analyses can fill
some of the gaps. Their research methods are common for understanding Earth’s
volcanic activity, but this is the first time those methods have been used
for other bodies in the solar system. With the ratios of sulfur dioxide,
sulfur monoxide and sulfur detected in the volcanic plumes of Pele and
other volcanoes on Io, Zolotov and Fegley used thermodynamic equations
to determine the redox state of Io’s magma at different volcanoes. Their
work suggests that oxidation processes commenced in Io’s interior after
initial gravitational separation, reducing the iron that had been present
in the primitive mantle region. “Our work suggests that there is no metal
iron in the mantle,” Zolotov says.
“It is very exciting that this type of modeling can be done remotely,”
says Alfred McEwen of the University of Arizona’s Lunar and Planetary Laboratory.
The exceptionally high-temperature magmas detected on Io have not been
common on Earth for billions of years. Galileo observations suggest
that Io’s magmatic temperatures may be even higher than Zolotov and Fegley
suggest. By studying the processes on Io, scientists hope to gain a better
understanding of how the interior evolution of Earth produced massive flood
basalts similar to the Columbia River Basalt Group in the northwestern
United States. “It’s like taking a look at ancient Earth,” McEwen says.
Laura Wright
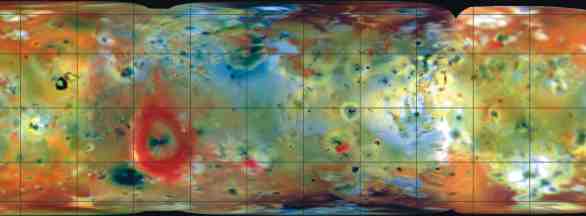
| Right: Composite photo of Io
shows an area 11,420 kilometers wide. Sulfurous materials appear in shades
of white, gray, yellow and brown. The bright red ring left of center is
Pele’s volcanic plume. Black spots mark recent volcanic activity and very
high temperatures. Image compiled in September 1996. NASA |
 |


