Geotimes

Untitled Document

Trends
Next Best Friend: Cultured
Diamonds
Megan Sever
Conventional thinking about diamonds may soon be changing. Diamonds —
long prized for their beauty, rarity and long generation times — are now
being created in a matter of hours in laboratories. What that change will do
to the diamond gem industry or to the market value of natural diamonds is still
in question. But what the lab-created diamonds could do for technology has the
science community buzzing.
Diamond as a semiconductor
 More than
80 percent of natural, mined diamonds are used for industrial purposes, as cutting
tools or abrasives for grinding and polishing other gemstones, metal, granite
and glass. The use of diamond as a semiconductor requires the highest purity,
best crystallinity and the introduction of electrically active atoms to create
the electrical pathways of the device. But almost all natural diamonds are unsuitable
for electronics due to defects, impurities and poor structure. Even gem-quality
natural and created diamonds, while valuable, may not be suitable as semiconductors
because of trace impurities. Only the purest of these stones are usable in high-powered
electronic applications from cell phones and personal computers to secure communication
lines.
More than
80 percent of natural, mined diamonds are used for industrial purposes, as cutting
tools or abrasives for grinding and polishing other gemstones, metal, granite
and glass. The use of diamond as a semiconductor requires the highest purity,
best crystallinity and the introduction of electrically active atoms to create
the electrical pathways of the device. But almost all natural diamonds are unsuitable
for electronics due to defects, impurities and poor structure. Even gem-quality
natural and created diamonds, while valuable, may not be suitable as semiconductors
because of trace impurities. Only the purest of these stones are usable in high-powered
electronic applications from cell phones and personal computers to secure communication
lines.
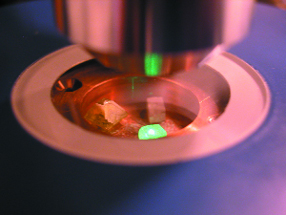
At the Antwerp High Diamond Council laboratory,
scientists study diamonds using a Raman/photoluminescence spectrometer to determine
whether a stone is synthetic or has been synthetically treated. Courtesy of
HRD.
Historically, there have been three major barriers to using natural diamonds
for electronic applications, says James Butler, a research chemist at the Naval
Research Laboratory. Natural diamonds have always been prohibitively expensive
for widespread use, and it is hard to find enough pure, large stones. Furthermore,
he says, no two stones are exactly alike and individual properties in each diamond
can cause problems in circuits. The final barrier to the widespread use of diamonds
for computer and electronic use has been the need for two types of diamonds
for electrical conductivity — p-type and n-type stones.
Both p-type and n-type semiconducting diamonds are needed for complex devices,
Butler says. But n-type diamonds do not exist naturally, and p-type diamonds
— blue diamonds — are so rare that there has been no economical way
to use them. Synthetically created diamonds, however, are removing those barriers.
For example, by “doping” a diamond with boron as it is being created,
“we can make p-type blue diamonds,” says Robert Linares, founder of
Apollo Diamond. Similarly, by doping colorless diamonds with phosphorus, scientists
can create n-type diamonds, he says. For semiconductor use in powerful electronic
devices, “we need a combination of the two diamond types in layers, sometimes
even layered with colorless diamonds as well,” Linares says. (Colorless
pure diamonds are actually insulators rather than conductors.)
Many semiconductors, like silicon, are used in the wide range of electronic
devices now available. But diamond, with its significantly higher heat tolerance
and speed, “is the second best semiconductor in the world, second only
to vacuum,” Butler says, and could create entirely new types of powerful
electronic devices — especially now that labs can grow pure and purposefully
“impure” stones at command. While the use of diamond in electronics
is probably still a few decades away, he says, it will change the semiconducting
industry.
Creating a diamond
In nature, diamonds crystallize under high pressures deep within Earth over
long periods of time. In the lab, two distinct processes can create diamonds
in much shorter periods of time. The high-pressure high-temperature (HPHT) process
essentially mimics nature’s process for creating diamonds, while chemical
vapor deposition (CVD) does the exact opposite. Instead of pressurizing carbon
into creating diamonds, CVD frees carbon atoms to allow them to join together
to create a diamond.
The two techniques were first explored in the 1950s. Because the first people
to suggest the creation of diamonds without high pressures were “laughed
away from the table,” the CVD technology is “still in its infancy,”
says Butler, who has been working on creating diamonds using CVD for 17 years.
Both processes quickly grow gem-quality diamonds, but ultimately, he says, the
CVD process is going to be most useful in electronic technologies because its
impurities and size can be easily controlled.
CVD begins with a tiny diamond seed that is placed in a vacuum. “Then we
flow hydrogen gas and methane into the vacuum,” says Linares, whose company
uses the technique. Plasma splits the hydrogen gas into atomic hydrogen, which
then reacts with the methane to produce a methyl radical and hydrogen atoms.
The methyl radical attaches to the diamond seed to grow the diamond. CVD diamond
growth is a linear process, Linares says, which means that the only limiting
factors in size are “how big the seed we start with is and how long we
leave it in the machine.”
HPHT also begins with a tiny diamond seed. In washing-machine-sized diamond
growth chambers, each seed is bathed in a solution of graphite and a metal-based
catalyst at very high temperatures and pressures, says David Hellier, chief
marketing officer at Gemesis, a company that uses HPHT. “Under highly controlled
conditions, the small diamond seed begins to grow, molecule by molecule, layer
by layer, emulating nature’s process,” Hellier says.
While General Electric pioneered this diamond-creation process and has since
been selling HPHT-created diamonds for industrial uses, the diamonds were not
sold as gemstones until Gemesis simplified the process and was able to create
much higher quality diamonds, Hellier says.
Selling cultured diamonds
| Diamond futures
The intrinsic properties of pure diamond — an excellent electrical
insulator and conductor that is the hardest and stiffest material known
— make it a natural for industrial and electronic uses. In the next
50 years, says James Butler, a diamond research chemist at the Naval Research
Laboratory, diamonds will likely appear in any number of electronic devices,
replacing silicon as the semiconductor of choice.
Some possible uses include the following:
* high-voltage and high-powered electronics, such as high-speed trains;
* high-frequency devices, such as high-powered radar and cellular base
stations;
* micro- and nano-electromechanical devices, such as clocks and filters
for cell phones;
* quantum computing, such as for secure communications;
* energetic radiation detectors, including medical dosimeters;
* high-powered lasers and optics, such
as in cable and telephone lines and as windows on space shuttles;
* and corrosion-resistant diamond electrodes, which could clean contaminated
environments.
|
Both Apollo Diamond and Gemesis are now selling created gem-quality diamonds
commercially. These “cultured” diamonds sell for significantly less
money than natural diamonds. Since 2003, Gemesis has been selling synthetic
“fancy-colored” diamonds at prices that are one-fourth to one-fifth
of the price of natural fancy-colored stones of comparable color, clarity, cut
and carat weight, Hellier says. High-quality fancy-colored diamonds make up
a small and highly lucrative part of the diamond industry. Exceedingly rare
and thus much more expensive than their colorless counterparts, these diamonds
range in color from red and pink, to blue and green and even bright yellow and
orange, depending on the impurities.
Apollo Diamond has taken a different route, selling colorless stones, though
the company will soon sell blue, pink and black diamonds as well, Linares says.
The diamonds produced can be very high quality: Even machines built by the diamond
industry to distinguish synthetic from natural stones can have trouble telling
them apart, which has some major diamond sellers in the industry scrambling.
Diamond labs in Belgium and elsewhere that have traditionally analyzed and certified
diamonds larger than one carat for color and clarity are now being asked to
distinguish between natural and synthetic or artificially colored diamonds.
“Our job is to protect the diamond community by finding methods to detect
synthetics and treated diamonds,” says Jef Van Royen, a physicist at the
Antwerp High Diamond Council in Belgium. “And with our current technologies,
we are quite confident that we can identify synthetics and treatments. But it’s
not a perfect science, especially as the diamond-growth and treatment technologies
get better.”
The Antwerp lab and a few others around the world primarily employ two types
of machines to detect created diamonds. The first shines light through the diamond
and analyzes the spectral characteristics of the absorbed or emitted light.
If it finds indications of a synthetic diamond, the labs use a secondary machine
that utilizes ultraviolet light to reveal the crystal’s inner structure,
Van Royen says. These machines examine defects in the diamonds, even microscopic
or atomic.
“Here we examine the growth structures of the diamonds,” Van Royen
continues. Diamonds are just like trees, he says, with growth rings surrounding
an inner core. Diamonds that are lab-created or treated (to change the color
of a natural stone) exhibit a different structure. Thus, while labs that use
these machines can distinguish created from natural diamonds, the worry in the
diamond industry is that people without these machines will not be able to detect
synthetically created diamonds.
“The average consumer or even jeweler will not be able tell a difference,”
Van Royen says. And while the diamond industry, he says, has “no problem
with synthetic diamonds,” they want to ensure that the created diamonds
are clearly labeled so that consumers know what they are getting.
Both Gemesis and Apollo are working to ensure the authenticity of their cultured
stones, according to Hellier and Linares. For example, all Gemesis cultured
diamonds greater than 0.25 carat are laser-inscribed with the Gemesis name and
a serial number. And all stones greater than 1 carat are accompanied by a certificate
of authenticity from the European Gemological Laboratory USA. But the question
remains, Van Royen says, whether everyone who eventually creates diamonds will
be as conscientious.
In the end, “we expect that synthetic diamonds will probably have a market
niche of their own,” Van Royen says. And some in the diamond industry think
that’s not a bad thing. “From a public policy perspective, the more
product types, selections, price points and competition, the better the market,”
says Martin Rapaport, chairman of the Rapaport Group, a network of companies
involved in the diamond industry. “There is a reasonable chance that we
can double the size of the diamond jewelry industry within the foreseeable future.”
But ultimately, the gemstone sales are only a means to an end, Linares says.
The big payoff, he says, will be in industrial technologies.
Untitled Document

 More than
80 percent of natural, mined diamonds are used for industrial purposes, as cutting
tools or abrasives for grinding and polishing other gemstones, metal, granite
and glass. The use of diamond as a semiconductor requires the highest purity,
best crystallinity and the introduction of electrically active atoms to create
the electrical pathways of the device. But almost all natural diamonds are unsuitable
for electronics due to defects, impurities and poor structure. Even gem-quality
natural and created diamonds, while valuable, may not be suitable as semiconductors
because of trace impurities. Only the purest of these stones are usable in high-powered
electronic applications from cell phones and personal computers to secure communication
lines.
More than
80 percent of natural, mined diamonds are used for industrial purposes, as cutting
tools or abrasives for grinding and polishing other gemstones, metal, granite
and glass. The use of diamond as a semiconductor requires the highest purity,
best crystallinity and the introduction of electrically active atoms to create
the electrical pathways of the device. But almost all natural diamonds are unsuitable
for electronics due to defects, impurities and poor structure. Even gem-quality
natural and created diamonds, while valuable, may not be suitable as semiconductors
because of trace impurities. Only the purest of these stones are usable in high-powered
electronic applications from cell phones and personal computers to secure communication
lines. 
