
Geotimes Home | AGI Home | Information Services | Geoscience Education | Public Policy | Programs | Publications | Careers

| Estimates are that human activity emits 7 billion
tons of carbon dioxide a year (see the feature on page 16 in this issue).
One proposed method for reducing how much of the greenhouse gas ends up
in the atmosphere is to store the carbon dioxide underground. Natural reservoirs
of the gas exist, suggesting that geologic carbon sequestration is feasible. For the past few years, two projects have, combined, been burying 2 million metric tons per year of man-made carbon dioxide instead of sending it into the atmosphere. And researchers in several countries are investigating other options for geologic storage of the greenhouse gas. One of the main goals of these studies is to verify that the gas can in fact remain buried for at least hundreds of years. “Not only is geologic [sequestration] showing the potential to account for most of the storage,but also, we already have the experience from the oil and gas industry in dealing with geologic formations and wells,” says Scott Klara, product manager for the carbon sequestration program in the U.S. Department of Energy’s National Energy Technology Laboratory. “Geologic will be the first line of defense in sequestration.” That experience from the petroleum industry is a key tool for monitoring the carbon dioxide after it’s injected into the ground. Seismic surveys, for example, are the means for monitoring sequestration beneath Statoil’s Sleipner natural gas field in the North Sea and EnCana’s Weyburn oil field in Saskatchewan, Canada, the largest projects actively sequestering carbon dioxide in geologic formations today. One of the biggest challenges for the long term, Klara says, will be finding ways to verify that any buried carbon dioxide is staying put — that is, monitoring for leaks. If large amounts of the greenhouse gas are buried in geologic formations, enough small leaks over time could undo the advantages of sequestration, he says. “Catastrophic levels are going to be easy to find and really are improbable,” Klara says. The small leaks and seepage that can happen through microfractures present the largest monitoring challenge, he adds. Public perception is also a factor. In Texas, a team with the Bureau of Economic Geology will inject 3,000 tons of carbon dioxide just below an abandoned oil field this summer. As part of their planning, says Susan Hovorka, one of the researchers on the project, they have been talking with nearby residents. “We’re working with local people to make sure they’re comfortable with it,” she says. Hovorka is confident in the geologic understanding her team has of the injection site. Geologists, she says, must make that confidence more robust and communicate it. “We have to show that the geologic understanding of the subsurface is accurate enough. We have an intuition that it’s accurate enough. We have to demonstrate it. … Mostly we have to bring out our understanding to the people.” Kristina Bartlett Back to top |
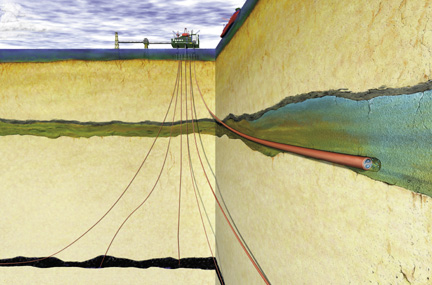 This summer,
a caravan of trucks will travel from the BP oil refinery in Texas City, Texas,
to an abandoned oil field east of Houston. The trucks will deliver a commodity
that is both valuable and troublesome: carbon dioxide. A team of scientists will
inject the carbon dioxide 5,000 feet deep into the Frio Formation, a saline aquifer
that sits above the depleted oil reservoir. Then they will watch what happens,
using seismic tools and tracers fielded by the Lawrence Berkeley and Oak Ridge
national laboratories.
This summer,
a caravan of trucks will travel from the BP oil refinery in Texas City, Texas,
to an abandoned oil field east of Houston. The trucks will deliver a commodity
that is both valuable and troublesome: carbon dioxide. A team of scientists will
inject the carbon dioxide 5,000 feet deep into the Frio Formation, a saline aquifer
that sits above the depleted oil reservoir. Then they will watch what happens,
using seismic tools and tracers fielded by the Lawrence Berkeley and Oak Ridge
national laboratories. The Great Plains Synfuels plant in Beulah, N.D., has been creating natural
gas from coal since 1984. The process of converting coal to natural gas releases
carbon dioxide, and until 1999 the plant released the greenhouse gas into the
atmosphere. “We didn’t have anywhere else to put it,” says Floyd
Robb, a spokesperson for Dakota Gasification Co., which runs the plant.
Now, the carbon dioxide is a commodity that has created a new revenue source
for Dakota Gasification. For the last 25 years, oil companies have used compressed
carbon dioxide to extract additional oil from reservoirs that were considered
depleted. They usually tap natural underground accumulations of carbon dioxide,
such as the Bravo Dome in New Mexico and McElmo Dome in Colorado. “Because
you can use carbon dioxide for oil recovery, it’s a natural process for
people to start saying, ‘What if we use man-made CO2?’”
says Scott Klara, product manager for carbon sequestration at the U.S. Department
of Energy’s National Energy Technology Laboratory, which researches technologies
for carbon sequestration.
Carbon dioxide injected into a depleted oil reservoir dissolves into the remaining
oil. In the process, it lowers the viscosity of the oil, making it easier to
extract. At the same time, some of the oil remains, sequestering much of the
dissolved carbon dioxide with it.
Robb says Dakota Gasification was looking for an oil producer nearby that might
want their carbon dioxide. They finally found one in the company PanCanadian
Energy Corp., now EnCana Corp., which has been running the Weyburn oil field
just over the border in Saskatchewan since 1955.
Dakota Gasification made a large capital investment to add a carbon dioxide
compressor to the plant’s process, as well as to build a 200-mile pipeline
that would bring the compressed carbon dioxide to Weyburn. Even with this investment,
Robb says, selling the carbon dioxide to EnCana is a significant revenue stream.
He says the company has added taps along the pipeline in North Dakota, anticipating
that oil fields in the state will eventually need the carbon dioxide for secondary
oil recovery.
EnCana reports that the injection and recovery project, which started in 2000,
will stretch the life of the oil field by 25 years, and that it is the sixth
largest recovery project in the world.
Today, about 1 million metric tons of carbon dioxide each year are going from
the gasification plant into the Weyburn field, Klara says. The field is sequestering
about 40 percent of what is produced at the Synfuels plant, says Malcolm Wilson
of the Petroleum Technology Research Centre at the University of Regina in Saskatchewan.
Over the next 10 to 15 years, EnCana will probably inject 18 million tons of
carbon dioxide into its 48-year-old Weyburn oil field in Saskatchewan, Wilson
adds. “Virtually all of that will ultimately remain in the reservoir,”
he says. “Our current view is that CO2 will remain down there
almost indefinitely. Any leakage will be small. The bulk of it, we anticipate,
will remain in the reservoir.”
This prediction is based on several factors, Wilson says, including likelihood
of earthquakes in the area (not much); likelihood of future mining activities
(a possibility because potash and salt lie beneath the oil field); likelihood
of small leaks through natural fractures; and, most importantly, likelihood
that the gas will escape through the more than 1,000 oil wells dotting the 70-square-mile
field. “It’s a big pin cushion,” Wilson says.
DOE and the Petroleum Technology Research Centre are part of a large coalition
of Canadian, U.S. and European research organizations, including the International
Energy Agency Greenhouse Gas R & D Program in the UK. The coalition is working
to monitor the fate of the injected carbon dioxide. “This is a natural
system so there are obviously going to be a host of anomalies,” Wilson
says. But, he adds, within a reasonable realm of predictability, the group —
using seismic surveys taken before and after injection started — is seeing
close matches between how the carbon dioxide is moving and how their models
suggested it would move. “It’s an old field so we have a huge amount
of information on it,” Wilson adds.
Kristina Bartlett
 Coal seams are
an appealing option because of their economic value, says Scott Reeves, executive
vice president for Advanced Resources International, an oil and gas consulting
firm. The injection of carbon dioxide into coal seams can actually enhance recovery
of coalbed methane, a key natural gas resource. “By using coal seams and
enhancing methane recovery, it lowers the cost because you’ve got the value-added
site benefit of the incremental methane recovery,” Reeves says. “If
you try to inject it, for example, into an aquifer, there’s no added benefit;
there’s just cost.”
Coal seams are
an appealing option because of their economic value, says Scott Reeves, executive
vice president for Advanced Resources International, an oil and gas consulting
firm. The injection of carbon dioxide into coal seams can actually enhance recovery
of coalbed methane, a key natural gas resource. “By using coal seams and
enhancing methane recovery, it lowers the cost because you’ve got the value-added
site benefit of the incremental methane recovery,” Reeves says. “If
you try to inject it, for example, into an aquifer, there’s no added benefit;
there’s just cost.”Nature has the best track record for sequestering carbon dioxide from the air
into the ground, through the process of weathering. Carbon dioxide is slightly
acidic and as it reacts with rocks and soil, it converts into other chemical
forms. The only problem in putting nature to work on carbon sequestration is
that the process takes too long by human standards. In order to help limit the
amount of carbon dioxide in the atmosphere, some geologists are looking to speed
the weathering process up through industrial means — converting carbon
dioxide into carbonate rocks.
“We end up making rocks,” says Klaus Lackner of the Earth Engineering
Center at Columbia University. But they have to start with rocks first. To do
so, they use magnesium silicates, a class of peridotite rocks that include serpentine
and olivine. Exposing magnesium silicate to an aqueous solution of the slightly
acidic carbon dioxide forms carbonate and silicate, such as sand. Presto-chango,
the carbon dioxide is gone and new carbonates and silicates have replaced the
original rock. And the process is exothermic, producing heat. “So its thermodynamics
are downhill, it happens spontaneously,” Lackner explains. This is why
weathering in nature also occurs over time.
So why aren’t we mass-producing carbonate rocks with our abundance of carbon
dioxide? Again, time is the limiting factor. The world has an abundance of magnesium
silicate rocks, but reacting those rocks with only carbon dioxide is a slow
process. “We are trying to take the process and accelerate it for an industrial
setting,” Lackner says.
In order to speed the reaction up, a stronger acid is also needed and, in some
cases, additional heat. The Albany Research Center in Oregon, and Ohio State
University, are both working on building cost-efficient methods.
Ultimately, achieving large-scale sequestration will mean building power plants
at magnesium silicate mines around the world that would convert the olivine
and serpentine into carbonates. The newly formed carbonates would then be put
back into the mines for permanent disposal.
The Ohio group is fine-tuning their high-pressure, high-temperature, three-phase
fluidized bed reactor, an apparatus that uses a mixture of acids to dissolve
serpentine in an aqueous solution of carbon dioxide.
“In 30 minutes we can convert about 25 percent of solid magnesium silicate
to carbonate at 1,000 [pounds per square inch] pressure and 80 degrees Celsius,”
says Ah-Hyung Alissa Park, lead author on a presentation about this technique
at the American Institute of Chemical Engineers in November. “At higher
temperatures and pressures the conversion rate goes up.”
Still, the science is in its infancy, Lackner says. “It is an example of
where we learn more the cleverer and better we will get.”
Christina Reed
Back to top
| The
National Energy Technology Laboratory Curt M. White, Ken LaSota, Richard J. Jones Global warming is currently a topic of major interest. Emission of anthropogenic greenhouse gases, such as carbon dioxide from fossil fuel utilization, has been associated with global warming. Multipronged strategies to reduce anthropogenic carbon dioxide emissions include both conservation and increased efficiency. The sequestration of anthropogenic carbon dioxide in underground and oceanic geologic settings is viewed by some as a viable long-term mechanism to reduce atmospheric carbon dioxide levels. The U.S. Department of Energy's National Energy Technology Laboratory (NETL) has developed a Focus Area where CO2 capture and sequestration in geologic formations and in the deep ocean are being studied. The report, Carbon Sequestration Research and Development, a road mapping document, guides our framing of the overall approach to the work, and is a source to focus individual research projects on specific goals. The report, published in December 1999, was prepared by more than 70 scientists and engineers, using input from more than 200 of their peers, and thus, represents a comprehensive guide to research and development activities and knowledge gaps in the carbon sequestration science arena. The vision stated in the report is to "possess the scientific understanding of carbon sequestration and develop to the point of deployment those options that ensure environmentally acceptable sequestration to reduce anthropogenic carbon dioxide emissions and/or atmospheric concentrations." The stated goal is to "have the potential to sequester a significant fraction of 1 gigaton of carbon per year in 2025 and 4 gigatons of carbon per year in 2050." Consequently, the goal of the NETL Carbon Sequestration Science Focus Area is to provide the enabling science and engineering to make that vision a reality. The primary goal of NETL's Focus Area is to develop, and evaluate, to the point of deployment, environmentally acceptable approaches to capture and geologically sequester carbon dioxide. A more recent report, Carbon Sequestration Technology Roadmap was used to further refine and direct the work. The overarching goal of these efforts is to assure safe, long-term sequestration of carbon dioxide. Carbon sequestration science is a relatively new field. It is remarkably
broad-based, encompassing major parts of chemistry, physics, biological
and geological sciences, as well as engineering, computational science,
and other disciplines. NETL's Carbon Sequestration Science Focus Area
divides its efforts into four major tasks: 1) Capture and Separation,
2) Geological Sequestration, 3) Oceanic Sequestration, and 4) Geological
Sequestration Modeling. NETL's Carbon Sequestration Science Focus Area
does not currently address other research areas, such as, sequestration
in terrestrial ecosystems and biological sequestration. At NETL a variety of projects are underway, each intending to acquire the knowledge required to advance one of the major components needed to make geologic sequestration of anthropogenic carbon dioxide a reality. Individual projects are attempting to model and simulate the geological sequestration process in terms of the chemical, physical, and geologic parameters involved in sequestration. Part of the modeling effort, for example, hopes to gain a better understanding of the behavior of carbon dioxide in porous media such as sandstone reservoir rock. In addition, a variety of experimental projects are underway to determine the parameters required to understand the dynamics of sequestration, data that are essential to the sequestration models and simulations being developed and analyzed by NETL. Investigation of the chemical interactions among carbon dioxide/water/rock is a constant theme throughout the Carbon Sequestration Science Focus Area. It pervades the Geological Sequestration, Oceanic Sequestration, and Geological Sequestration Modeling tasks. Developing a comprehensive understanding of the formation of Ca/Mg carbonates by the reaction of carbon dioxide with minerals, or of carbon dioxide with water to form carbonate anion, bicarbonate anion, and carbonic acid, and their subsequent reactions with minerals or brine, either above or below ground, is vital to much of the work. The kinetics of these reactions must be better defined. The interaction of carbon dioxide and sea water to form hydrates is the major emphasis of the work in the oceanic sequestration task. A project entitled An Investigation of CO2/Water/Rock Interactions and
Chemistry seeks to develop insight into what occurs on a chemical/mineralogical
level when large volumes of carbon dioxide are pumped into brine-bearing
formations. This project addresses the aqueous chemistry of carbon dioxide
with brines and rock. With the U.S. Geological Survey's (USGS) Hydrothermal
Laboratory as our partner, NETL is investigating the uncertainties associated
with the heterogeneous reactions that may occur with different minerals
and strata, as well as the uncertainties associated with the complex ionic
equilibria and kinetics of interactions among carbon dioxide, water and
rock. A substantial research effort is directed at experimental and modeling investigations of coal seam sequestration. Specifically, we are studying the environmental factors that affect the ability of coal to adsorb carbon dioxide. The Focus Area has investigated the adsorption isotherms of carbon dioxide on the Argonne premium coals. NETL seeks to more clearly define the effects of a variety of coal seam properties such as temperature, pressure, pH of water associated with the seam, and coal rank on the ability of the coal to adsorb carbon dioxide. A new theory has been developed based upon coal swelling that results in improved fits of both our observed experimental results and results from the literature. This new hypothesis will be incorporated in the reservoir simulators being developed for enhanced coalbed methane (ECBM) production. Another aspect of this project is focused upon estimating the interlaboratory comparability of carbon dioxiode isotherm results obtained on the Argonne premium coals. Two laboratories outside the United States - one at The Netherlands Institute for Applied Geoscience, and the second in Australia at the CSIRO - along with four laboratories in the United States, have agreed to measure the carbon dioxide isotherms on the Argonne coals. This should be a useful exercise in estimating the interlaboratory comparability of such measurements. Additionally, NETL is preparing a comprehensive review article on sequestration of carbon dioxide in coal seams. Before preparation of the review article was initiated, NETL assembled a bibliography of sequestration-related articles. This bibliography was constructed using commercially available bibliographic software, Reference Manager. NETL intends to make the bibliography available to the public in the near future. NETL is planning to begin construction of a unique facility, a Geological
Sequestration Core Flow Laboratory (GSCFL), late in 2002. NETL is designing
a flexible state-of-the-art GSCFL where geotechnical properties and chemical
reactions can be investigated for a variety of geological formations into
which carbon dioxide can be injected. The goal is to be able to simulate
the conditions found in all the major categories of potential geological
sequestration sites including oil and gas fields, deep unmineable coal-seams,
brine formations, and natural gas hydrates. The facility will be designed
to accommodate a variety of confining pressures and controlling temperatures
for long term experiments while monitoring host rock pore-fluid chemistry.
The facility will be designed so that permeability changes can also be
monitored continuously during flow. The final design criteria for a facility
that can test rock types under a variety of controlled conditions and
environments are now being developed. Ultimately, the experimentally derived
databases obtained using the GSCFL will provide information on the geotechnical
effects and chemical interactions that occur when carbon dioxide is injected
into natural rock strata with similar geological and geotechnical properties
as those tested at the GSCFL. These results will then be compared with
those predicted by modeling experiments, and will ultimately be used to
improve the models. Closely linking the laboratory, field, and modeling
activities in an iterative relationship will ensure accurate results and
maximize progress. The GSCFL will be capable of housing a variety of rock
materials, of different sizes and configurations, and will be fully instrumented
to record real-time and pre-and post-conditions of the samples during
experimentation. When fully developed, the GSCFL will be equipped with
a scanning electron microscope, ion chromatograph, XRD, ICP-MS, magnetic
resonance imaging, and computer aided tomography, as well as traditional
instruments used in petrographic analysis, among others. Advances in high-speed computing and improved understanding of chemical
behavior and fluid flow in porous media permit the use of simulations
and modeling as tools for designing, optimizing, analyzing, and better
understanding of chemical and physical processes. The Geological Sequestration
Modeling task integrates computational science capabilities within the
Carbon Sequestration Science Focus Area, building upon the solid foundation
of experimental research. It complements and supports the laboratory and
field work, and promotes a more thorough understanding of the fundamental
science. The major emphasis of the laboratory effort in the Carbon Sequestration
Science Focus Area is on geological sequestration and capture technologies.
Accordingly, a complementary suite of computational science capabilities
is being developed in these areas as well. A holistic approach (consisting
of laboratory and modeling and simulation studies conducted in concert)
to acquiring the fundamental body of knowledge required to successfully
take carbon sequestration to fruition is being undertaken. |
 |
Geotimes Home | AGI Home | Information Services | Geoscience Education | Public Policy | Programs | Publications | Careers |