Geotimes

Untitled Document

Energy & Resources
Fusion stalemate
A "Takeout" nuclear plant
Mineral of the Month: Indium
Fusion
stalemate
 The mere possibility that fusion could produce a viable energy source for the
future has scientists and policy-makers chomping at the bit to get projects
under way. However, the major international collaborative project, ITER, has
been stalemated over where to build its facilities since December 2003, with
a decision deadline past and another fast approaching. In the meantime, the
U.S. fusion project, FIRE, has been shelved until a decision is reached on the
international project. But with both projects expected to take at least 10 years
to get going once approved, “the time to act is now,” says Dale Meade,
a plasma physicist with the Princeton Plasma Physics Laboratory in New Jersey,
and director of FIRE.
The mere possibility that fusion could produce a viable energy source for the
future has scientists and policy-makers chomping at the bit to get projects
under way. However, the major international collaborative project, ITER, has
been stalemated over where to build its facilities since December 2003, with
a decision deadline past and another fast approaching. In the meantime, the
U.S. fusion project, FIRE, has been shelved until a decision is reached on the
international project. But with both projects expected to take at least 10 years
to get going once approved, “the time to act is now,” says Dale Meade,
a plasma physicist with the Princeton Plasma Physics Laboratory in New Jersey,
and director of FIRE.
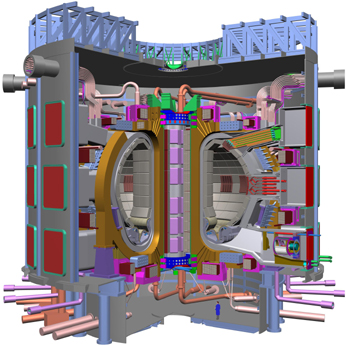
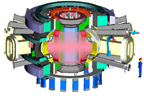
Two programs -- ITER, an international
collaboration, and FIRE, a U.S. program -- are designed to develop the technology
to create fusion power through burning plasma science. Neither project has been
built yet. Plans for the ITER device (far right), which will weigh approximately
19,000 metric tons, and FIRE (right), which will weigh about 1,400 metric tons,
are shown here. The human figures shown for scale are both 2 meters tall. Images
courtesy of Dale Meade, Princeton Plasma Physics Laboratory.
“We need to find out if fusion can be an energy source for the future,”
Meade says. Currently, fusion energy is in the research and development phase
and has not yet been developed to test for feasibility.
Proponents of fusion power bill it as an environmentally clean, safe and inexhaustible
energy source that can operate continuously to meet surging demand, according
to the Department of Energy (DOE). The idea is to recreate the nuclear fusion
reaction that happens on the sun, when its plasma particles collide at high
temperatures and release energy. Fusion does not produce highly radioactive
waste like that from nuclear fission power plants. Originally called the International
Thermonuclear Experimental Reactor, ITER was designed to provide the know-how
to build the first fusion power plant and is a collaborative effort between
the United States, Russia, China, South Korea, Japan and the European Union.
Construction of the international project will cost about $5 billion over 10
years, says Anne Davies, associate director of DOE’s Fusion Energy Sciences.
FIRE, the U.S.-only fusion program, was designed to be a lower-cost approach
to using burning plasma to produce fusion energy, Meade says. It is significantly
smaller than ITER, costs less than $1 billion to build and is more directly
aimed at feasibility — determining the scientific requirements for attaining
and controlling a fusion fire. ITER has a stronger emphasis on technology development.
“FIRE was designed to be a fall-back way of producing a burning plasma,
in case ITER failed to go forward,” Davies says. But “even if we were
to build FIRE, we would still need ITER in the future,” she says. The programs
“fill different needs,” even if they are similar in scientific scope.
Thus, currently, the United States has focused funding on the international
project and has shelved FIRE, Davies says. But several deadlines have passed
for resolving the current deadlock with ITER — over where to build the
facility, in France or Japan — and little progress has been made. Both
Japan and France have added incentives to get the six partners to agree to their
locale, says Bill Spears, a spokesman for ITER. In late September, the European
Council of Ministers increased the pressure by calling for agreement on France
as the site by a Nov. 25 meeting, with “as many partners as possible.”
The most recent international meeting was at the end of October.
“I can’t really project a time-scale for resolution, but I do believe
it will happen,” Davies says. “We don’t know what fusion will
bring or if it will even work. But the potential is so great that we have to
find out.”
Megan Sever
Back to top
A
“Takeout” nuclear plant
Imagine a nuclear power plant that can be deployed via truck or ship to a small city,
where it provides light, heat and hot water, and is then disposable once the
fuel is spent. The idea is not so far-fetched: Scientists are now developing
just such a “takeout” nuclear power plant.
Nuclear energy provides 20 percent of the electricity used in the United States
and 16 percent worldwide, and is expected to grow as the energy demand grows,
especially in developing nations, according to the Department of Energy (DOE).
Thus, DOE’s Lawrence Livermore National Laboratory (LLNL) in Livermore,
Calif., along with U.S. and international partners in industry and universities,
are looking for ways to meet that growing need. They say the answer is a “small,
sealed, transportable, autonomous reactor” (SSTAR), a self-contained nuclear
reactor in a tamper-resistant container.
Traditional reactors are water-cooled and supply up to 1,000 megawatts of energy.
But they are gargantuan in proportion to the SSTARs: At only 15 meters tall
by 3 meters wide, SSTARs are designed to fit a different niche, says Bill Halsey,
a nuclear engineer at LLNL.
While each SSTAR supplies only 10 to 100 megawatts of energy, they produce less
waste and are ideal for locales where human and physical infrastructure cannot
support a large power plant, such as remote locations or developing nations,
he says.
The reactor core, which is about the size of a desk, would sit inside a vessel
circulated and cooled by liquid lead — “basically a big, deep bathtub”
60 feet underground, Halsey says. For 30 years, uranium and plutonium in the
SSTAR’s reactor core would provide energy. Then, the core would be removed
and shipped back to the United States, where waste would be recycled or stored.
During the core’s lifetime, little if any maintenance or work would be
needed, which would eliminate the need for large crews and lower the operating
costs. Inherent safety features and no access to the fuel make it “melt-down
proof” and “terrorist-proof,” Halsey says. “Even if we lose
all outside controls, the reactor can shut itself down safely,” he says.
Part of DOE’s rationale is that by having the United States design and
deploy the reactors, this program would eliminate the need for developing countries
to develop their own nuclear programs, thus helping global nonproliferation
goals.
Before this program can be implemented, however, it still faces a number of
challenges, Halsey says, such as figuring out how to control the oxygen in the
reactor so that the liquid lead does not corrode the metal core, and how to
package and transport the reactors safely. “If they wanted to, industries
could deploy a simplified system like this today,” Halsey says, but “it’s
probably going to be 10, 20 or 30 years” before the program is really market-ready.
Megan Sever
Back to top
Mineral
of the Month: Indium
U.S. Geological Survey Commodity Specialist
Micheal W. George has prepared the following information on indium — a
rare metal used in coatings on television and computer screens.
Indium was discovered in Germany in 1863. Although it is a lustrous silver-white
color, the finders named the new material for the “indigo” spectral
lines the mineral created on the spectrograph. Indium ranks 61st in abundance
in Earth’s crust and is about three times more abundant than silver or
mercury.
The earliest known use of indium was in 1934, when small amounts were added
to certain types of gold dental alloys. Demand for indium continued to increase
during World War II and later for use in nuclear control rods.
Fifty years ago, indium played a key role in the development of the first transistor
radios, ushering in a new age of consumer electronics. Now, indium coatings
are used to defog aircraft and locomotive windshields and to keep glass doors
on commercial refrigerators and freezers frost-free. Indium also is at the forefront
of advanced technology, as a small but critical element in liquid crystal displays
(LCDs).
Indium oxide with 10 percent tin oxide (indium tin oxide) is an ideal film for
converting data from electrical to optical form in flat-panel displays for televisions
and computers because it exhibits good heat reflectivity, electrical conductivity
and optical transparency. The use of indium in thin film for LCDs is increasing
at an exponential rate and is garnering media attention because of the possibility
of indium shortages.
Currently, more than 70 percent of indium consumed in the world is used in such
thin-film products. Several companies in Southeast Asia have announced that
they are not only opening new plants, but that they also are increasing the
size of the glass to make bigger displays.
Indium (used in small amounts) has also been essential to recent breakthroughs
in light-emitting diode (LED) technologies for lighting and electrical displays.
LED lights are resistant to mechanical shock, temperature changes and have a
long life. The renovation of the illumination of the Thomas Jefferson Memorial,
in Washington, D.C., used 17,000 LEDs, reducing electrical requirements by 80
percent, using 20 percent fewer fixtures and increasing lighted areas by more
than 30 percent.
Most indium is recovered as a byproduct of zinc refining and, to a lesser extent,
from the refining of tin. As of 2003, the world reserve base for indium was
an estimated 6,000 tons.
Worldwide indium demand in 2003 exceeded primary production, which was estimated
at 250 tons. With world inventories nearly exhausted, and the world demand for
indium estimated at 450 tons in 2003, recycling is expected to increase. Currently,
50 percent of consumed indium tin oxide is from recycled sources, but the share
from recycled sources could be as high as 75 percent in 2004.
For more information on indium visit minerals.usgs.gov/minerals.
Back to top
Untitled Document


 The mere possibility that fusion could produce a viable energy source for the
future has scientists and policy-makers chomping at the bit to get projects
under way. However, the major international collaborative project, ITER, has
been stalemated over where to build its facilities since December 2003, with
a decision deadline past and another fast approaching. In the meantime, the
U.S. fusion project, FIRE, has been shelved until a decision is reached on the
international project. But with both projects expected to take at least 10 years
to get going once approved, “the time to act is now,” says Dale Meade,
a plasma physicist with the Princeton Plasma Physics Laboratory in New Jersey,
and director of FIRE.
The mere possibility that fusion could produce a viable energy source for the
future has scientists and policy-makers chomping at the bit to get projects
under way. However, the major international collaborative project, ITER, has
been stalemated over where to build its facilities since December 2003, with
a decision deadline past and another fast approaching. In the meantime, the
U.S. fusion project, FIRE, has been shelved until a decision is reached on the
international project. But with both projects expected to take at least 10 years
to get going once approved, “the time to act is now,” says Dale Meade,
a plasma physicist with the Princeton Plasma Physics Laboratory in New Jersey,
and director of FIRE.
