|
NEWS NOTES — NEWS
Geochemistry
Ice twists under pressure
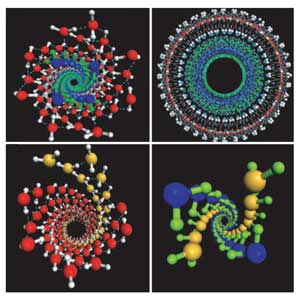 |
| Squeezed into tiny carbon nanotubes and subjected to pressures 40,000 times Earth’s atmosphere, ice responds by twisting from its familiar six-sided snowflake structure into DNA-like spirals. Image is courtesy of Xiao Cheng Zeng. |
Falling snowflakes may come in countless forms, but under the right conditions, water can freeze into shapes never seen during any winter. Confined inside tiny, hollow cylinders called carbon nanotubes, and subjected to high pressures similar to those found at a planet’s core, water freezes into tiny ice spirals that resemble the DNA double helix, a new study shows.
Understanding what drives the ice to twist itself into spirals could offer clues to how other self-assembling molecules and structures, like proteins, organize themselves — and could give scientists a new form of water to hunt for on other planets, such as the icy moons of Jupiter.
Scientists know of 15 forms of ice in nature, with structures ranging from cubic to rectangular to rhombic. The shapes that ice crystals take depend on the angles between their hydrogen and oxygen atoms, which are connected by chemical bonds. Most of the ice on Earth’s surface and in its atmosphere, known as Ice-1, takes the familiar hexagonal shape of snowflakes. In the much lower temperatures found high in the atmosphere, however, ice crystals can also appear as tiny cubes.
The other 13 forms of ice only occur at high pressures, and those forms are the most likely to occur on the icy moons of Jupiter or other outer planets, says Xiao Cheng Zeng, a chemist at the University of Nebraska at Lincoln. “We were curious how water behaves in those extreme environments,” he says.
Zeng and his team wanted to determine what might happen to ice subjected not only to high pressures but also packed in a tiny, tightly confined space, conditions such as might be found within an icy planet’s core. To do this, the team simulated water freezing at high pressures inside hollow carbon nanotubes, which are often employed by chemists as useful “nano-test tubes,” he says. Carbon nanotubes are also hydrophobic, or water-repellent, which helps constrain the hydrogen bonds that bind ice molecules.
Reporting the Dec. 26 Proceedings of the National Academy of Sciences, the team found that as the pressure increased from 500 million Pascals to 4 billion Pascals (40,000 times the pressure of Earth’s atmosphere), the ice inside the nanotubes assembled into six different structures. At some pressures, the ice formed one of four variations on a multi-layered, tube-within-a-tube structure, similar to structures previously observed in ice at normal atmospheric pressures. At the highest pressures, however, the ice threw a curve: When subjected to pressures more than 40,000 times that of Earth’s atmosphere, the ice twisted into a double-walled helical structure, a never-before-seen shape that Zeng’s team dubbed an “ice helix” or “spiral nano-ice.”
The discovery is a “big surprise” that highlights the “amazing variety of forms that this supposedly simple material of water takes on,” says David Oxtoby, a chemist at Pomona College. Although so far only observed in the laboratory, the ice helixes could exist naturally in constrained environments such as the outer planets of the solar system, he says. “It’s the sort of thing where once you start to look for it maybe you’ll find it.”
In addition to suggesting what astronomers might discover on Jupiter’s icy moons, the spiral shape shows how the ice “adapts to the environment, to the confinement and pressure,” and offers chemists a broader window into how materials can self-assemble and organize themselves under extreme conditions, Zeng says. Understanding such processes could help guide how scientists construct new materials, he says.

 Subscribe
Subscribe


