|
FEATURE
Seeding the Sea
Erin Wayman
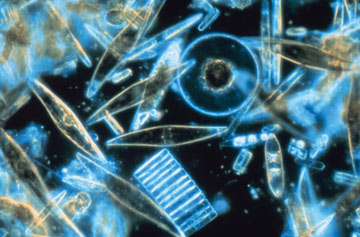 NSF Polar Programs/Prof. Gordon T. Taylor, Stony Brook University |
| Diatoms, a common type of phytoplankton, live in the oceans surrounding Antarctica. |
 Karen Selph, University of Hawaii |
Phytoplankton lead a brief, but important life. Collectively, these microscopic sea “plants” remove carbon from the ocean’s surface to make food, releasing half of Earth’s oxygen along the way. Many then become lunch for critters higher up the food chain. Others simply die out and sink as part of a slurry of decaying matter that bacteria eat. Some of the slurry, including fecal matter from organisms that eat phytoplankton, escapes the bacteria and sinks hundreds of meters deeper. There in the ocean’s dark depths it remains for a century or longer until ocean currents slowly recycle the icy water back to the surface. A smaller percentage journeys all the way to the seafloor and becomes incorporated into the sediment and eventually the geologic bedrock, laying the foundation for future oil, natural gas or coal deposits.
Because phytoplankton carry carbon to the deep ocean when they die, society is now calling upon them to help solve the cascading greenhouse gas problem.
Scientists predict that climate change caused by carbon dioxide and other greenhouse gases will affect everything from weather patterns to agriculture to the world’s economy. Altering nature caused the problem, and now some say altering nature further can fix it. Climos, a San Francisco-based company, is betting on that philosophy. The company’s leaders think they can help fix the planet’s carbon problem through ocean iron fertilization.
Ocean iron fertilization takes advantage of the ocean’s part in the carbon cycle. To carry out photosynthesis, phytoplankton need iron, a nutrient that is rare in some parts of the ocean. By “fertilizing” these patches of the sea with iron, Climos plans to stimulate phytoplankton growth and create large blooms of the tiny plants. The blooms should remove dissolved carbon from the sea, eventually storing it deep in the ocean: If the carbon reaches at least 500 meters, it will not resurface again for 100 years. The ocean then draws down carbon dioxide from the atmosphere to replenish its carbon levels.
The plan sounds straightforward, but there is a problem. Scientists do not know how much carbon ocean fertilization can sequester in the long term. Although experiments show that adding iron to the ocean stimulates phytoplankton blooms, researchers cannot say with certainty how much carbon dioxide these blooms take in, how much makes it to the deep ocean or how long it stays there. “The experiments done to date have not been designed to test that,” says Kenneth Coale, director of the Moss Landing Marine Laboratories in Moss Landing, Calif.
Perhaps more worrisome than whether ocean fertilization can sequester enough carbon dioxide to make a dent in the world’s greenhouse gas levels is how ocean fertilization may affect ocean ecosystems. Artificial phytoplankton blooms could affect organisms all the way up the food chain, scientists warn, and result in everything from the production of other greenhouse gases to the depletion of oxygen. And because the oceans have complex water circulation patterns, changes in one fertilized patch near Antarctica could end up affecting an ecosystem thousands of kilometers away.
Sorting out the answers to these unresolved questions has become a pressing matter due to the recent emergence of carbon emissions trading; just last year, companies bought and sold $64 billion worth of carbon credits. Now companies like Climos want to sell carbon credits based on the carbon they sequester. Climos hopes to start conducting fertilization experiments as soon as 2009. And if the company can demonstrate that it works, Climos founder and CEO Dan Whaley says, it will sell credits from those experiments.
 Kenneth Coale, MLML |
| Seawater from the Southern Ocean (left) and seawater collected from a patch in the Southern Ocean after scientists enriched it with iron to create a phytoplankton bloom (right). |
Selling such carbon credits before lingering uncertainties are resolved has some scientists worried. “It’s premature to apply for carbon credits from the next generation of experiments,” says Philip Boyd, a biogeochemist at the University of Otago in New Zealand. Ken Buesseler, a biogeochemist at the Woods Hole Oceanographic Institution in Woods Hole, Mass., agrees. “I don’t think we’re there yet.”
Just add iron
In the late 1980s, John Martin, an oceanographer at Moss Landing Marine Laboratories, pieced together the relationship between phytoplankton and iron. He noted several areas in the ocean where phytoplankton seemingly had all the nutrients they needed to thrive, but were not flourishing. He proposed that a lack of iron — which enters the ocean from land by wind-swept dust — was the problem. He also studied paleoclimate records from the past 500,000 years, which show that when the atmosphere is rich in iron-laden dust, carbon dioxide levels decrease. The correlation led Martin to famously joke, “Give me half a tanker of iron, and I’ll give you an ice age.”
Since then, dozens of scientists have cruised around the world, pouring tons of dissolved iron into the ocean. No ice ages followed. But these experiments validated Martin’s hunch that a lack of iron limits phytoplankton populations, and sparked debate on whether artificial iron fertilization could mitigate global warming.
The first validation came a few months after Martin died in 1993. Researchers from Moss Landing Marine Laboratories organized Iron Experiment I. They sailed to a 64-square-kilometer patch of ocean 500 kilometers south of the Galapagos Islands and seeded the sea with iron. During the nine-day experiment, phytoplankton biomass doubled. In the last 15 years, scientists have conducted 11 similar experiments in other iron-limited regions of the equatorial Pacific, northern Pacific and Southern oceans. In these experiments, scientists investigated ocean patches no larger than a couple hundred square kilometers for up to several weeks. In all cases, adding iron to these waters fueled phytoplankton blooms.
These experiments also demonstrated that the oceans draw down carbon dioxide in response to the flourishing blooms. But it is not clear what happens next, Coale says. Most experiments did not observe the most important stage of the phytoplankton bloom: its demise, when phytoplankton die and the carbon sinks toward the ocean’s bottom.
 Kenneth Coale, MLML |
| Researchers aboard the R/V Melville recover phytoplankton samples during the 2002 Southern Iron Ocean Experiment. |
But a few experiments did follow the carbon, using radioisotope tracers and sediment traps that collect sinking organic matter. Based on the available data, in January, 16 scientists suggested in a commentary in Science that commercial ocean fertilization would sequester no more than several hundred million metric tons of carbon each year, compared to the 6.5 billion metric tons of carbon humans release into the atmosphere annually. In 2004, however, members of the European Iron Fertilization Experiment fertilized an ocean eddy in the Southern Ocean and reportedly observed much more carbon sinking toward the deep ocean than any previous experiment, but the results are not yet published.
Extrapolating estimates of carbon sequestration for commercial ocean fertilization from previous experiments is tricky. “It’s difficult to scale up” the experiments, Boyd says, because things do not scale linearly. “At the moment,” he says, “models give us the best windows of what will happen in the future.”
The models do not look promising for ocean fertilization schemes. Jorge Sarmiento of Princeton University in New Jersey has used the results of iron fertilization experiments to model how much carbon could be sequestered through ocean fertilization. If the entire Southern Ocean were continuously fertilized for a century, Sarmiento says, only about six parts per million of atmospheric carbon dioxide would be sequestered over the entire period. Other published models suggest long-term fertilization could result in higher values, ranging from 15 up to 107 parts per million. The total concentration of carbon dioxide in the atmosphere today is 385 parts per million.
Yet Margaret Leinen, who left her post as assistant director of geosciences at the National Science Foundation to be Climos’ chief science officer, points out that even modelers have a hard time saying what would happen after larger fertilizations because researchers rely on data from experiments that were not designed to provide the data needed to make such models.
Indeed, “the studies done up until now have been done on a very small scale,” says Andrew Watson, a biogeochemist at the University of East Anglia in the United Kingdom. Furthermore, these studies “can’t follow what’s happened over a long time period.” Now, new experiments on the order of 1,000 to 10,000 square kilometers or larger that observe blooms from start to finish are needed, Buesseler says, tracking carbon as it descends into the deep ocean.
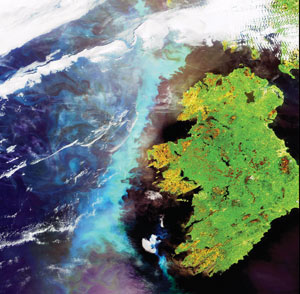 ESA |
| Natural phytoplankton blooms (in turquoise) off the coast of Ireland (above), Norway (below) and Namibia (far below). |
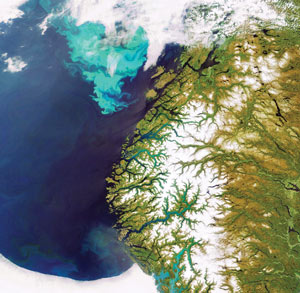 ESA |
 ESA |
That’s exactly what Climos intends to do. The company wants to test a method of ocean fertilization that it is developing sometime in mid- to late 2009 and has talked with the U.S. Environmental Protection Agency about the legality of releasing large amounts of iron into the ocean from an American ship. Although Climos has not yet chosen a test location, Whaley and Leinen say the company will fertilize a 10,000-square-kilometer patch and observe it for at least 60 to 70 days. They are not alone. India also plans to fund a large fertilization experiment in the Southern Ocean next year.
Iron’s downside
One reason ocean fertilization may be an attractive solution to climate change is that the oceans are more capable of absorbing carbon than land is, says Brian Von Herzen, founder of The Climate Foundation, an organization that supports ocean fertilization research and the development of free-range aquaculture. By some estimates, the oceans have already soaked up about a third of the world’s anthropogenic carbon. But some scientists question how much more they can handle. “To absorb that anthropogenic footprint may be beyond the oceans’ capability without detrimental effects,” Coale says.
One potential problem is that ocean fertilization may ultimately “rob” other areas of their nutrients, Boyd says. Phytoplankton gobble up not only iron but all sorts of nutrients in the sea. Over-stimulating phytoplankton growth could strip an ocean patch of all of its nutrients. And because of what Boyd calls the “tyranny of ocean circulation,” this could cheat ocean life far away. For example, waters from the Southern Ocean feed upwellings farther north in the tropics, which nourish the sea life there, Sarmiento says.
Another problem, Coale says, is that ocean fertilization could actually lead to the production of more greenhouse gases. The deeper layers of the ocean act as a “liquid compost pile,” he says. If too much decaying phytoplankton descends deep, the bacteria that decompose the ocean’s biomass might generate a lot more nitrous oxide and methane — greenhouse gases that are more potent than carbon dioxide — and possibly negate any benefits achieved through carbon sequestration. The bacteria would also use up a lot of the water’s oxygen. As with nutrients, oxygen-depleted water gets re-circulated elsewhere, Coale says, where it could harm ocean wildlife such as fish.
Coale also notes, however, that ocean fertilization might provide the ocean’s inhabitants with some relief from ocean acidification. As more anthropogenic carbon enters the oceans, the oceans become more acidic, to the detriment of delicate organisms such as corals and crustaceans. If phytoplankton blooms absorb carbon from the water, however, the ocean could become less acidic. During some phytoplankton blooms, Coale says, scientists have measured high pH levels in the ocean, corresponding to less acidity.
But to keep the ocean safe for its inhabitants, quantifying — and anticipating — any negative consequence is crucial if commercial ocean fertilization is to move forward. Von Herzen suggests that companies should tend to their seeded sea patches just as farmers tend their fields. Long-term monitoring should be required, he says. But John Cullen, a biological oceanographer at Dalhousie University in Nova Scotia, Canada, worries that science may not be up to the challenge. “We have to be able to predict downstream effects, how big they are and provide measurements to verify predictions,” Cullen says. “There’s a limit to how accurately we can predict alterations over great areas of the ocean.”
Iron’s future
Commercial ocean fertilization faces more than just scientific challenges. Last November, the Scientific Groups of the London Convention and London Protocol — global treaties that regulate dumping waste into the open ocean — issued a “Statement of Concern” regarding ocean fertilization. They concluded that scientific knowledge to date does not justify commercial fertilizations.
In May, the United Nations Convention on Biological Diversity, of which the United States is not a member, echoed that concern. That same month, parties of the London Convention and London Protocol met to discuss the issue. Climos and the rest of the world are now waiting to hear the outcome of the London Convention’s meeting.
Legal issues aside, ocean fertilization is an attractive carbon sequestration method because it’s cheaper than other carbon sequestration methods, Leinen says. But under the Kyoto Protocol, it is not an acceptable carbon offset project. Things could change after Kyoto expires in 2012, she says. But as more companies and individuals get on the green bandwagon, Climos might do well in the voluntary carbon market. Google, for example, invested an unspecified amount in carbon offset projects last year to reduce its carbon footprint.
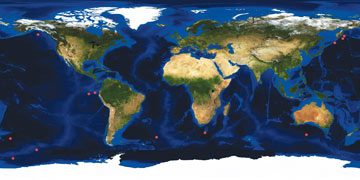 AGI/NASA |
| Locations (red dots) where scientists have conducted iron enrichment experiments. |
Climos intends for its ocean fertilization method to comply with the precedents established by the Kyoto Protocol’s Clean Development Mechanism, which specifies a project must verify it has sequestered carbon for at least 100 years. “That’s a tough thing to prove,” Coale says of an ocean fertilization project. “We do have the technology that can measure carbon flux, but you would have to track it 300 to 500 meters into the water column” to ensure it will not resurface for at least a century.
Scientists will have an opportunity to weigh in on Climos’ ocean fertilization plans later this year. In the fall, the company will host a series of ocean fertilization workshops where it will work to arrive at a consensus on a method for its first fertilization demonstration project. Once Climos is ready to head to sea, it will recruit independent scientists to conduct the trial, Leinen says. The data will also be freely available on the Internet. “If you’re going to do something like this,” she says, “you need the highest level of transparency and responsible behavior.”
No matter how responsible Climos might be, ocean fertilization’s future is far from certain. “Done properly and in the right place it does sequester carbon,” Watson says, but “you can’t solve the greenhouse gas problem with it.” Proponents of ocean fertilization understand that. But “we’re in such dire straights,” Von Herzen says, “that we can’t afford to let any scalable option slide off the table.”

 Subscribe
Subscribe


