|
NEWS NOTES
Nitrogen: Too much of a good thing
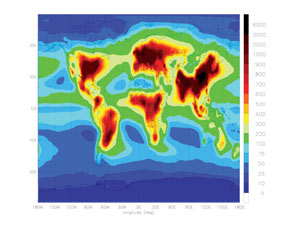 Courtesy of James Galloway, as originally published in Dentener et al., 2006. |
| This map shows estimated nitrogen deposition from nitrogen emissions around the world. Dark red areas have the highest nitrogen levels and dark blue areas have the lowest. |
Nitrogen is essential to life. But nitrogen can also do harm — usually when humans unleash too much of it into the environment. Now is the time to get serious about curtailing our use of nitrogen, scientists warn. That means, among other things, changing how we use fossil fuels and fertilizers.
“We want people to be aware that we’re perturbing the global nitrogen cycle in the same way we’re perturbing the carbon cycle,” says Doug Capone, a marine microbiologist at the University of Southern California in Los Angeles and co-author of one of two papers about nitrogen that appeared May 16 in Science.
Plants and animals need nitrogen to make DNA and build proteins. But 99 percent of the world’s nitrogen is in a form that plants and animals can’t use. Certain soil bacteria transform this nitrogen into a usable form called reactive nitrogen, which combines with other elements to make ammonia, nitrous oxide, nitrate and many other compounds.
Humans have also found ways to create reactive nitrogen, mainly for crop fertilizers. “Our ability to make synthetic fertilizers is one of the greatest public health benefits of all time,” says Alan Townsend, a biogeochemist at the University of Colorado at Boulder and co-author of one of the papers. Without fertilizers, Townsend says, farmers would have trouble growing enough food to feed the world.
But only 30 to 50 percent of the nitrogen in fertilizers is taken up by plants, says James Galloway, a biogeochemist at the University of Virginia in Charlottesville and lead author of one of the papers. The rest can escape into the environment — either by leaking into groundwater and then traveling through rivers to coastal areas or by being converted into gases that are released into the air. Burning fossil fuels also introduces reactive nitrogen into the atmosphere.
Once reactive nitrogen is on the loose, it causes a lot of damage. In coastal waters, nitrogen run-off can over-stimulate the growth of microorganisms, leading to algal blooms or oxygen-deprived “dead zones.” In the atmosphere, reactive nitrogen compounds lead to smog and haze, acid rain and the thinning of the ozone layer. Furthermore, as a greenhouse gas, nitrous oxide is 300 times more efficient at trapping heat than carbon dioxide is, Capone says.
A portion of Earth’s human-derived reactive nitrogen also ends up in the open ocean, Robert Duce, a marine and atmospheric chemist at Texas A&M University in College Station, and his colleagues report in one of the papers in Science. About 80 percent of the reactive nitrogen that reaches the ocean from the atmosphere is human-made, they say. That extra nitrogen “fertilizes” new biological production, leading to growing numbers of plankton that utilize large amounts of bicarbonate from the ocean’s surface. In response, the ocean draws down more carbon dioxide from the atmosphere to restore its carbon dioxide/bicarbonate balance. About 10 percent of the ocean’s yearly drawdown of human-derived carbon dioxide, the researchers estimate, is the result of artificial nitrogen fertilization.
This may sound like a good carbon sequestration scheme, but the extra nitrogen in the oceans leads the oceans to emit more nitrous oxide into the air — offsetting about two-thirds of the benefits gained by the ocean’s carbon dioxide drawdown, Duce and his colleagues suggest. Duce says these are still “very early estimates.” But he adds that excess nitrogen in the oceans “may play a role in climate change,” he says. “Models need to take these factors into consideration.”
In the other Science paper, Galloway and his colleagues point out several strategies that could reduce reactive nitrogen levels. These strategies include decreasing nitrogen oxide emissions from burning fossil fuels and improving the world’s access to sewage treatment, through which bacteria transform reactive nitrogen back to its harmless form.
Changing agricultural practices, however, will have the largest impact, Townsend and Galloway say. Farmers need to improve their crops’ nitrogen uptake, for example, by matching the timing and amount of fertilization with the plants’ needs, says Kenneth Cassman, an agronomy professor at the University of Nebraska in Lincoln who did not work on either paper. This requires farmers to monitor soil conditions more closely or use controlled-release fertilizers — choices that are more time-intensive and costly, Cassman says.
Outlining these strategies is a good first step, says Cheryl Palm, chair of the International Nitrogen Initiative, an organization committed to optimizing the benefits and minimizing the negative effects of nitrogen. The next step, she says, is to develop nitrogen-reduction strategies specific to different regions’ sources of nitrogen into the environment and then evaluate these strategies from an economic standpoint.
Links:
Is the nitrogen cycle out of whack?, Geotimes online, Videocast, May 20, 2008

 Subscribe
Subscribe


