|
Trends and Innovations
Taking a Fresh Look at Desalination
Despite its location along the edge of the seemingly endless Pacific Ocean, Southern California is facing a water crisis. In recent years, available freshwater resources in this region have been pushed to their limit by population growth, overconsumption, resource mismanagement, drought and pollution. Meanwhile, other places throughout the United States, such as Florida and the Southwest, have similar stories to tell.
Although scientists, industry and the government have, for decades, eyed desalination as a potential means of producing consumable freshwater from saltwater, economic and technical challenges have made the process impractical and kept it largely out of reach. But now, against the backdrop of a looming water crisis, efforts to make desalination feasible are growing, resulting in new technological developments.
Desalination is the process of producing freshwater by removing salt from seawater or brackish water. In 2003, Sandia National Laboratories and the U.S. Department of the Interior’s Bureau of Reclamation took a step toward increasing the role of desalination in the United States with their publication of the Desalination and Water Purification Technology Roadmap, which is slated to be updated again this year. The roadmap’s guiding vision is that “by 2020, desalination and water purification technologies will contribute significantly to ensuring a safe, sustainable, affordable, and adequate water supply for the United States.”
If that vision is to be realized, however, much work needs to be done: Currently, desalination’s contribution to the total U.S. water supply is “negligible,” says Peter Gleick, president of the Pacific Institute, an independent research organization in Oakland, Calif. Desalination plants exist in every state, but have the capacity to provide less than 0.4 percent of the water used in the United States, he says. And right now, industries — not municipalities — use the majority of the water produced by those plants.
 Historically, the primary reason desalination has not played a larger role in the water-supply portfolio of the United States has been its cost. Part of the hefty price tag is from the capital costs of building and maintaining a desalination plant, which account for 50 to 70 percent of the produced water’s cost, Gleick says.
Historically, the primary reason desalination has not played a larger role in the water-supply portfolio of the United States has been its cost. Part of the hefty price tag is from the capital costs of building and maintaining a desalination plant, which account for 50 to 70 percent of the produced water’s cost, Gleick says.
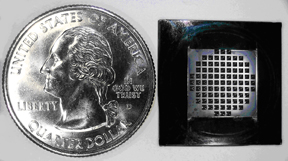
In the lab, nanotube membranes show promise for desalination applications by allowing water to move through rapidly while filtering out larger molecules. The filter unit, supported on a silicon chip and shown next to a quarter for scale, has 89 windows that each hold a membrane made from nanotubes embedded in a ceramic matrix. Photograph is by Hyung Gyu Park.
Another reason for the high cost is energy. Desalination processes, which are generally either membrane-based or thermal-based, consume large amounts of energy to build pressure, provide an electrical current or create heat. For example, a membrane-based process known as reverse osmosis uses pressure to force salty water through semi-permeable membranes, effectively filtering out the salts. Another membrane-based process, electrodialysis, uses an electrical current that forces salt ions through membranes, leaving freshwater behind. Thermal-based processes, on the other hand, use heat to distill freshwater from salty water.
In the past, the energy consumed by these operations typically accounted for 30 to 50 percent of the produced water’s cost, Gleick says. But new technologies and process developments, which focus mainly on membrane-based desalination — the preferred process in the United States — show promise for reducing the cost of desalination.
Earlier this year, for instance, a team of scientists at Lawrence Livermore National Laboratory in California developed a membrane that uses “nanotubes” to filter water much faster than most conventional desalination membranes. The nanotubes — tiny hollow tubes composed of carbon atoms — are vertically aligned throughout the membrane’s thin ceramic matrix. The chemical composition of each nanotube allows water to flow through rapidly, while its miniscule inner diameter — just six water molecules across — prevents the passage of larger molecules, such as salts. “Basically, for the same pore size and the same applied pressure” as a conventional membrane, “we get more water through,” says Olgica Bakajin, who led the research team.
 Thomas Mayer, project manager of the Advanced Concepts Desalination Program at Sandia, says the high flow rate of the new membranes is “exciting,” but that before the technology can be applied to desalination, the researchers will have to demonstrate that the new nanotube membranes can filter out salt as effectively as conventional membranes. If they can, and if implemented successfully on a commercial scale, the highly efficient membranes could reduce the energy costs of desalination by as much as 75 percent, the scientists estimate.
Thomas Mayer, project manager of the Advanced Concepts Desalination Program at Sandia, says the high flow rate of the new membranes is “exciting,” but that before the technology can be applied to desalination, the researchers will have to demonstrate that the new nanotube membranes can filter out salt as effectively as conventional membranes. If they can, and if implemented successfully on a commercial scale, the highly efficient membranes could reduce the energy costs of desalination by as much as 75 percent, the scientists estimate.
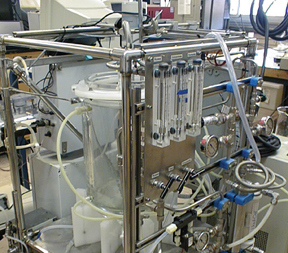
Researchers are working on ways to make desalination more efficient by developing processes that maximize the amount of freshwater recovered from salty water, such as this laboratory system, which is used to test high-recovery desalination. Photograph is by Yoram Cohen/PoleSep Research Laboratory at UCLA.
In other research, scientists at the Water Technology Research Center at the University of California in Los Angeles, have been testing a new process, called “accelerated precipitation softening” (APS), in combination with reverse osmosis as a means of maximizing the recovery of freshwater during desalination. Normally, only about 50 to 80 percent of the brackish water put into a desalination system is recovered; the remainder leaves the system as a highly salty wastewater product, or concentrate, that can be difficult and costly to dispose of.
In the APS process, which is currently optimized for brackish water, concentrate from an initial reverse-osmosis step is demineralized, filtered, and made more acidic before being treated again by reverse osmosis. This treatment of the concentrate prevents salt precipitates from building up and clogging desalination membranes during the second reverse-osmosis step. As a result, the process allows for the recovery of as much as 98 percent of freshwater and subsequently minimizes concentrate management issues, says Yoram Cohen, the center’s director, making it more cost-effective than current processes.
“It’s a good thing to do,” Mayer says of the process. But he cautions that while high-recovery processes may work well at preventing the buildup of certain salts, such as calcium carbonate and calcium sulfate, they may not be able to prevent the buildup of other materials that may be in the water, such as silica. Thus, he says, whether such a process will succeed at a particular geographic location depends to some degree on the makeup of the source water.
In another new effort, the Affordable Desalination Collaboration (ADC) — an organization composed of businesses, government agencies and water districts in Southern California — is working on a new membrane-based desalination system that uses existing commercially available technology. The system combines high-efficiency membranes and pumps with an ultra-high-efficiency ceramic energy recovery device “in an innovative way” to reduce energy consumption, says G.G. Pique, founding member of ADC and president of Energy Recovery, Inc., a desalination equipment supplier.
In a demonstration earlier this year, ADC’s system set a new desalination record by producing freshwater from seawater using only half the energy that a typical membrane system would have required just six years ago, and one-fifth less than the amount of energy currently required to pump river water to Southern California, Pique says. He hopes that ADC’s work will help dispel the perception that all desalination is expensive and that “it’s the Hummer” — that is, the energy consumer — of water production, he says.
Although advances in technology and processes should go a long way toward making it more affordable in the future, desalination still presents other challenges. Its potential environmental impacts are manifold: Intake pipes can draw in marine organisms, a plant can deplete inland aquifers, and highly concentrated effluents can cause contamination if not properly disposed of, for example.
Additionally, some officials worry that extensive desalination along dry coastal areas could spur poorly planned growth and development. Location is another concern, as communities may be resistant to having a desalination plant in their own backyard.
“The environmental and socio-political problems associated with desalination are probably more challenging than the technical problems,” Mayer says. “They need to be addressed first.”
Mayer adds that while desalination “should definitely be considered,” it should be adopted only after water conservation and resource management have been optimized. Gleick shares a similar view: “Desalination is an inevitable part of our water future,” he says, but in terms of solving our water supply problem, “it’s not a silver bullet.”
Jennifer Yauck

 Subscribe
Subscribe

