|
Web Extra Friday, January 11, 2008
Blues’ clues: UV light reveals diamonds’ fingerprints
 Chip Clark/Smithsonian |
| The fabled (and, some say, cursed) Hope Diamond is the world's largest blue diamond. |
Under the spotlight of its museum setting, the famed Hope Diamond shines with a brilliant blue sparkle. When exposed to ultraviolet (UV) rays, however, the blue diamond glows a deep red. For years, scientists were puzzled by this fiery glow, as its red color is apparently unique among blue diamonds, which tend to glow in shades of bluish-green or white light under UV rays. But an unusual study gathering data from dozens of the extremely rare gems now suggests that every natural blue diamond actually possesses its own unique glow — and that that glow can both distinguish individual diamonds, and tell the real from the fake.
The Hope Diamond was first mined in India and brought to France in 1668, where it became a crown jewel until it was stolen during the French Revolution and eventually surfaced, recut, in London in 1830. Bought by Henry Hope, a banking heir, it passed on through other wealthy families until donated to the Smithsonian National Museum of Natural History in Washington, D.C. in 1947 by jeweler Harry Winston. Along with its travels, the stone has carried with it enduring legends of a curse and doomed owners, which help draw millions of visitors a year to the Smithsonian.
But the diamond is more than a historic jewel, says Jeffrey Post, curator of the National Gem Collection at the Smithsonian. “Visitors tend to think of the Hope Diamond in terms of its human history,” Post says. “But it’s also a rare blue diamond, so it’s a very interesting natural history object as well.”
Wanting to understand the diamond’s unusual red glow, called phosphorescence, a team of researchers led by Sally Eaton-Magaña, a mineralogist now at the Gemological Institute of America in Carlsbad, Calif., and Post examined the diamond’s phosphorescence with a portable spectrometer. Measuring the wavelengths of light emitted by the diamond under UV rays, they discovered that the diamond glowed in not just one, but two colors: In addition to the red light (wavelengths of 660 nanometers), the diamond was emitting light with wavelengths at about 500 nanometers — in other words, bluish-green.
Under UV light — a part of the electromagnetic spectrum that is just outside the visible light spectrum, with wavelengths ranging from about 250 nanometers to 400 nanometers — materials such as diamonds absorb energy in the form of photons. That excites electrons in the diamond, kicking them up to a higher energy state. Eventually, the electrons fall back to their original state, releasing energy in the form of light as they fall. In fluorescent materials, such as some types of calcite, that light is released immediately, so that the object glows only as long as the UV rays continue to shine on it. In phosphorescent materials, such as blue diamonds, the glow lingers for some time, even after the light is shut off.
Curious to see how other blue diamonds’ phosphorescence measured up to the Hope’s, the researchers examined light spectra from the Blue Heart, also in the Smithsonian’s collection, as well as about 60 other natural blue diamonds. To the team’s surprise, Post says, each of the blue diamonds phosphoresced at about the same two wavelengths, although for most of the other blue diamonds the bluish-green light was more intense than the red — and therefore, the gem only appeared to glow blue-green. Additionally, the length of time of each diamond’s lingering glow varied from gem to gem, they reported in the January issue of Geology.
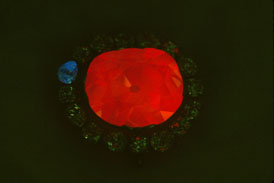 John Nels Hatelberg |
| Under ultraviolet light, the blue Hope Diamond glows a fiery red. The color and length of time that a blue diamond glows, or phosphoresces, is also a "fingerprint" that uniquely identifies the diamond. |
Initially, the scatter of phosphorescence intensities and length of glow time among the gems was curious, Post says. “Then it dawned on us that scatter can be helpful, in that each diamond has its own characteristic behavior. That gives us a way of assigning a marker to a particular blue diamond.”
Phosphorescence can also help distinguish natural blue from synthetic blue diamonds. The researchers also examined the spectra of synthetic blue diamonds, which are grown in laboratories and have the element boron added to the diamond structure to give the blue color. The spectra showed that under UV light, the synthetics only phosphoresce at the bluish-green wavelengths, not the red. That discovery could provide another useful tool to jewelers anxious to give customers more confidence in their gems, Post says. Additionally, the relatively portable and inexpensive nature of the technology — which allowed the researchers to visit the diamonds in their own homes, rather than riskily transporting them — allows it to be available to a larger group of people, he says.
Exactly what causes natural blue diamonds to phosphoresce at both wavelengths is still unclear, Post says. Trace amounts of boron in the diamond structure not only give the diamonds their blue color, but are likely connected to the phosphorescence as well, he says. The boron atoms pick up the electrons in their excited state before releasing them back to their original donor molecule. However, the two different wavelengths emitted suggest there are two different donor molecules, possibly two different forms of nitrogen, which is present in very tiny amounts in these diamonds.
The study adds “another piece to the armament” of tools that scientists can use to study diamonds and other gems, says George Harlow, curator of the “Nature of Diamonds” exhibit at the American Museum of Natural History in New York City. “This was their first stab” at examining these diamonds, and it was intriguing to see how the various features of the spectrum varied among them, Harlow says. Still, to really understand the diamonds’ differences would require a full suite of analyses of their structures and compositions — generally difficult to do on such valuable, rare gems, he says.
Part of the real value of the study, Harlow adds, is that it focuses on the Hope Diamond as a subject of research as well as legend. The Hope Diamond “has all kinds of legends and a curious history about its cut — there’s a lot of drama related to it. But this cultural icon is also a scientifically interesting object.”
Post agrees with that sentiment. “This study demonstrates its value as a research specimen, not just a historical object,” he says. “As a scientist, I find it nice to remind people of that.”
Links:
The Hope Diamond at the Smithsonian National Museum of Natural History
“Next Best Friend: Cultured Diamonds,” Geotimes, July 2004
“Fingerprinting a diamond's source,” Geotimes, December 2003
Back to top

 Subscribe
Subscribe


